Which Statement Best Describes an Oxidation Reduction Reaction
4- Symmetric ethers are generally synthesized by dehydration of alcohols. Which of the following would cause the greatest reduction of wildfires.
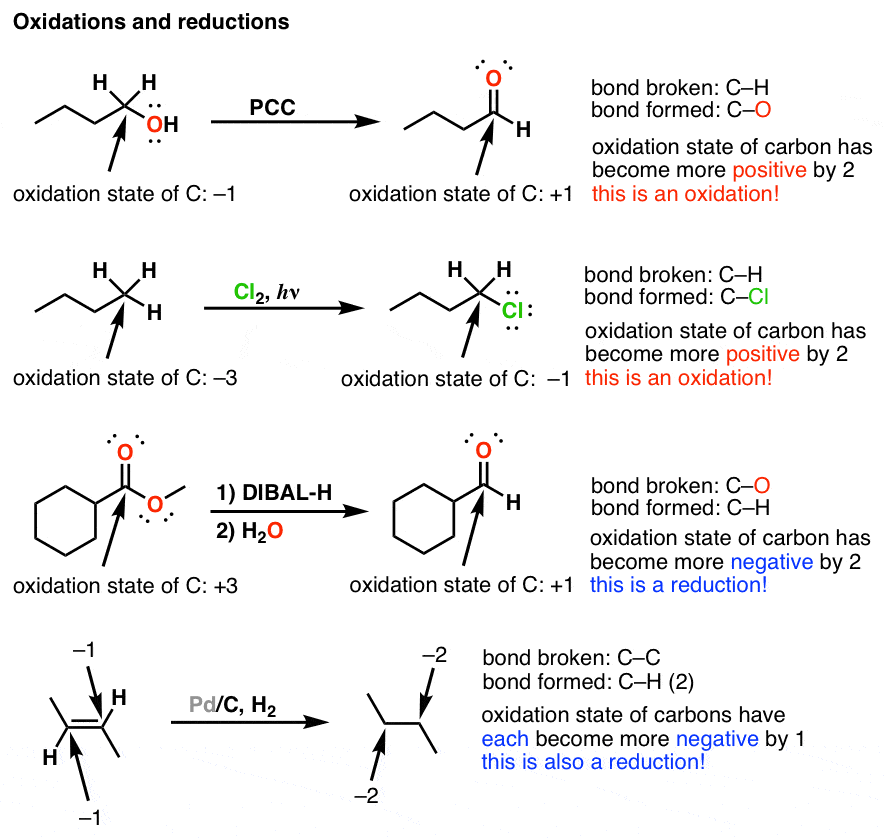
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry
A chemical reaction in which there are fewer products than reactants B.

. You might be interested in. O In this reaction both ethanol and NAD are oxidized. In this reaction iron is oxidized and copper is reduced.
NAD NADH-H NAD NADHH CH3CH2OH alcohol CH CHO CH3COOH ethanol dehydrogenase acetaldehyde HO acetic acid acetaldehyde dehydrogenase O This is not an oxidation-reduction reaction. The most common isotope of uranium has 92 protons and 146 neutrons. It occurs at the anode of a galvanic cell.
However an oxidizing agent oxidizes something else and gets reduced therefore gaining electrons. A chemical reaction in which electrons are released from the system D. The following equations are half reactions and reduction potentials.
This is not an oxidation-reduction reaction. Which of the following activities is inconsistent with drive-reduction theory. Click card to see definition Iron is being oxidized.
In this reaction iron is reduced and copper is oxidized. Decarboxylation mostly refers to a reaction of carboxylic acids erasing a carbon atom from a chain of carbons. 1- E1 describes an elimination reaction in which the rate-determining step does not involve the base.
Nataly_w 17 1 year ago. A chemical reaction that involves oxygen C. 4Al 302 2Al2O3 In this reaction aluminum is oxidized and oxygen is reduced In this reaction aluminum is reduced and oxygen is oxidized In this reaction th aluminum and oxygen are oxidized This is not an.
This is the basis of redox reactions. How many atom in protons. Which of the following statements best describes an oxidation-reduction reaction.
In this reaction iron is reduced and copper is oxidized In this reaction both iron and copper are oxidized Which statement best describes the following reaction. Which statement best describes a reduction reaction. Its atomic number is 14 and its atomic mass is 28.
A chemical reaction that involves oxygen C. Which statement best describes the following reaction. Now consider lithium LI and fluoride F2 as oxidizing agents.
Memorize flashcards and build a practice test to quiz yourself before your exam. The reduction potentials for Cl and Felt are. F2 g 2e- - 2F- aq has a reduction potential of 287 V.
A chemical reaction in which electrons are transferred between reactants 2Beryllium Be has four. The presence of which reactant is the best indicator of an oxidation-reduction reaction. This is a redox reaction in which octane C8H18 is oxidized.
It involves the gain of electrons. 1Which statement best describes an oxidation-reduction reaction. Which chemical formula shows the correct.
D Copper transfers two electrons to iron. It requires an oxidizing agent. Which answer best describes what is happening in the following reaction.
In this reaction both iron and copper are oxidized. Carboxylation is a completely reversible process which is the first chemical step in photosynthesis. Start studying the Oxidation-Reduction Quiz flashcards containing study terms like Which describes the oxidizing agent in a chemical reaction Given the reaction below which is the oxidized substance.
Fe Cu2 Fe2 Cu a. Question 2 Which statement BEST describes the following reaction. Fe---Fe2 2e- Which statement best describes what is taking place in this half reaction.
A chemical reaction that involves oxygen C. 1Which statement best describes an oxidation-reduction reaction. Redox reactions are characterized by the actual or formal transfer of electrons between chemical species most often with one species the reducing agent undergoing oxidation losing electrons while another species.
1Which statement best describes an oxidation-reduction reaction1 point Chemistry. A chemical reaction in which electrons are released from the system D. 2- Rate of formation of carbocations follow the order 3 2 1 CH3 3- Cyclohexanol is oxidized to ketone using Jones reagent.
2C8H18 25O2 16CO2 18H2O 6 What is the oxidation number for S in the compound SO3. Which of the following statements is true regarding pyruvate oxidation. Li aq e- - Li s has a reduction potential of -304 V.
1Which statement best describes an oxidation-reduction reaction1 point A. The original reaction by Tsutomu Mizoroki 1971 describes the coupling between iodobenzene and styrene in methanol to form stilbene at 120 C with potassium acetate base and palladium chloride catalysis. A chemical reaction in which electrons are released from the system D.
A chemical reaction in which there are. Which statement best describes what changes occur over the course of the following oxidation-reduction reaction. Fe Cu2 Fe2 Cu A Two electrons are lost.
If there is formation of a precipitate the reaction is an. 2Beryllium Be has four electrons. Recall that O has an oxidation number of -2 Electrons are transferred.
It involves the loss of electrons. Decarboxylation reaction is defined as a chemical reaction that eliminates a carboxyl group and liberates carbon dioxide CO 2. This work was an extension of earlier work by Fujiwara 1967 on the PdII-mediated coupling of arenes ArH and alkenes and earlier work by Heck 1969 on the.
A chemical reaction in which there are fewer products than reactants B. 5- The SN2 reaction occurs with inversion of configuration. A chemical reaction in which there are fewer products than reactants B.
It occurs when a non-metal ion becomes an element. But when an element is reduced it gains electrons. A chemical reaction in which electrons are transferred.
Which identifies an oxidation-reduction reaction. When an element is oxidized it loses electrons. 1Which statement best describes an oxidation-reduction reaction.
Cl2g 2e - 2 Claq E 136 V Felt le - Felt E 077 V Which of the. In this reaction both iron and copper are reduced. Redox reductionoxidation ˈ r ɛ d ɒ k s RED-oks ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of atoms are changed.
Mg Cl2 Mg2 2Cl Which best identifies why the rusting of an iron nail in the presence of water. Physical Chemistry Balancing oxidation-reduction equations 45 26 Reviews STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Consider the half reaction below. Alex Ar 27 Answer.
B Iron changes into copper. C Iron transfers two electrons to copper. Fluorine F has nine electrons.
A chemical reaction in which electrons are transferred between reactants. E Two electrons are gained. O O O If there is a change in the oxidation states of atorns from reactants to products the reaction is an oxidation- reduction reaction If there is formation of salt and water the reaction is an oxidation-reduction reaction.
Click again to see term 110.

Chemistry Reduction And Oxidation Reactions Wikiversity

Vitamin Water Label Template Elegant Vitamin Water Label Nutrition Facts Nutrition Facts Bla Label Templates Bottle Label Template Water Bottle Labels Template

1 Which Statement Best Describes An Oxidation Reduction Reaction 1 Point A A Chemical Reaction In Brainly Com
No comments for "Which Statement Best Describes an Oxidation Reduction Reaction"
Post a Comment